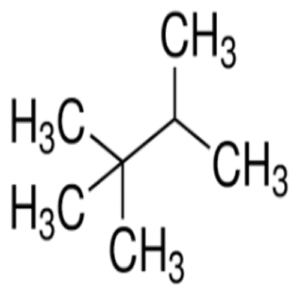
Triptane (2,2,3-Trimethylbutane): The High-Octane Hydrocarbon That Shaped Fuel Science
In the world of hydrocarbons and fuel chemistry, few compounds have earned a reputation as distinctive as Triptane, scientifically known as 2,2,3-Trimethylbutane. Though it may not be widely discussed outside chemical and engineering circles, Triptane has played an important role in the history of fuel performance, particularly in octane rating and engine research.
This article explores what Triptane is, why it matters, and where it fits into modern chemistry.
What Is Triptane?
Triptane is a highly branched alkane with the molecular formula C₇H₁₆, making it an isomer of heptane. Its IUPAC name, 2,2,3-Trimethylbutane, reflects its compact and symmetrical molecular structure.
Basic Chemical Information
- Chemical Formula: C₇H₁₆
- Molecular Weight: ~100.2 g/mol
- Chemical Class: Branched alkane
- Physical State: Colorless, volatile liquid
- Odor: Mild hydrocarbon smell
What makes Triptane special is not its size, but its extreme degree of branching, which strongly influences how it behaves during combustion.
Why Triptane Is Important
1. Exceptionally High Octane Performance
Triptane is famous for its very high octane number, meaning it resists engine knocking better than most hydrocarbons. Knocking occurs when fuel ignites prematurely in an engine cylinder, reducing efficiency and potentially damaging the engine.
Because of this property, Triptane was historically used as a reference compound in octane rating research, especially during the early and mid-20th century when fuel performance was critical for aviation and high-compression engines.
2. A Benchmark in Fuel Research
Although Triptane is not commonly used as a commercial fuel today, it remains valuable in:
- Fuel performance studies
- Combustion chemistry research
- Comparison testing for alternative fuels
Its predictable combustion behavior makes it ideal for laboratory and academic analysis.
Chemical Structure and Combustion Behavior
The key to Triptane’s performance lies in its branched molecular structure. Compared to straight-chain alkanes like n-heptane, branched molecules:
- Burn more smoothly
- Ignite more slowly
- Resist auto-ignition
This makes Triptane an excellent example of how molecular structure directly impacts fuel quality.
Industrial and Research Applications
While Triptane is not produced on a large commercial scale, it is still used in:
- Fuel chemistry research
- Octane calibration studies
- Hydrocarbon reaction modeling
- Educational demonstrations in organic chemistry
Its limited production is mainly due to the cost and complexity of synthesis compared to conventional fuels.
Safety and Handling
Like most low-molecular-weight alkanes, Triptane is:
- Highly flammable
- Volatile
- Potentially harmful if inhaled in high concentrations
Proper laboratory safety procedures, ventilation, and fire precautions are essential when handling this compound.
Triptane in Modern Context
With the rise of renewable fuels and cleaner energy technologies, Triptane is no longer a practical fuel option. However, its legacy remains important. It helped scientists understand the relationship between molecular structure and engine performance, knowledge that still guides modern fuel design today.
Conclusion
Triptane (2,2,3-Trimethylbutane) may be a small molecule, but its impact on fuel science is significant. As one of the most knock-resistant hydrocarbons ever studied, it stands as a classic example of how chemistry shapes technology.
For students, researchers, and fuel technologists, Triptane remains a fascinating compound that bridges organic chemistry and real-world engineering.